How can mechanical energy be convert to heat energy? What objects convert mechanical energy to heat energy? The mechanical equivalent of heat.
Rub your hands quickly together. Your hands warm up. Some of the work of rubbing has turned into heat. Benjamin Thompson was the first man to study the relationship between work and heat. He showed that you could get endless amounts of heat from friction. But Thompson never made measurements to find the exact amount of work needed for a given amount of heat.
From 1842 to 1870 James Joule conducted a series of work and heat experiments. Joule used an apparatus to determine the relation between mechanical energy and heat energy.
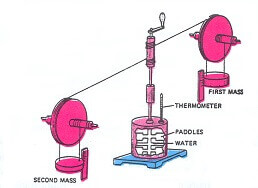
The work produced by the falling weights makes the paddle wheels turn. The wheels stir the water and the water gets warmer. This shows that work has been turned into heat. Joule measured this heat as the increase in temperature of water whose mass and specific heat were known.
- He found that 4. 2 joules of work are always changed into 1 calorie. (1 joule= 1 N.m)
- So, 1 cal= 4. 2 joules.
The conversion of work to heat is reversible. Heat can be changed to work, or other forms of energy. The devices that convert heat energy into work and other forms of energy are called heat engines. Car engines, steam engines and steam turbines are examples of heat engines.
Many heat engines get their heat from the burning of fuels such as coal, oil and natural gas. The engine converts some of the heat released into work. The rest of the heat is released into the environment. The efficiencies of heat engines are necessarily low.
Joule also used electricity to heat water. He found that the increase in the temperature of water depended on the amount of electric energy used. In some buildings, water is heated by the electricity.