What are the affects of temperature on solubility? Information on the temperature factor that affect solubility.
Affects Of Temperature On Solubility
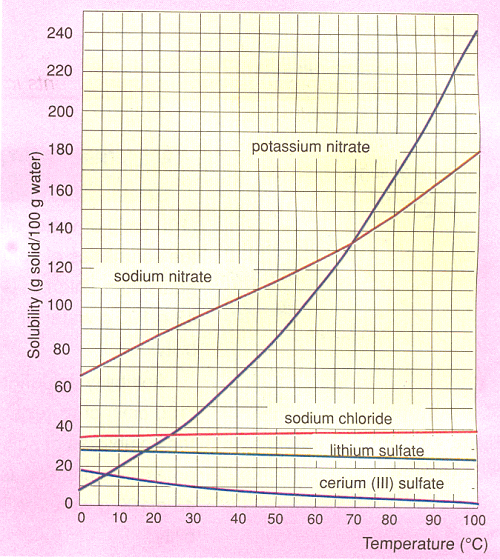
An increase in the temperature increases the kinetic energy of particles. An increase in the energy of the molecules increases the solubility of endothermic reactions. The solubility of most solids increases with increasing temperature. The solubility of exothermic reactions decreases with increasing temperature. Increasing the temperature decreases the solubility of some solids such as calcium carbonate, calcium sulfate. Figure 2 show the solubility as a function of temperature for some common substances, all plotted together in the same graph. These curves obviously show that the way the solubility of a substance changes with temperature is a distinctive property.
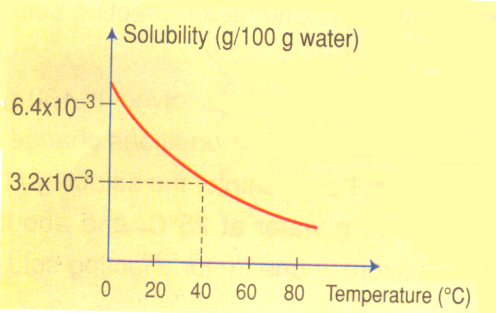
Gases may also dissolve in water. For example, fish living in water obtains the oxygen they need from oxygen dissolved in the water.
The solubility of gases in water usually decreases with increasing temperature. When water is heated in a beaker you can see bubbles of air forming on the side of the glass before the water boils.
As the temperature rises, the dissolved air molecules begin to “boil out” of the solution long before the water itself boils.On a hot summer day, an experienced fisherman usually picks a deep spot in the river or lake to cast the bait. Because the oxygen content is greater in the deeper, cooler region, most fish will be found there.
Solubilities of liquids also depend on temperature. If the boiling point of solute is higher than that of solvent, solubility of the solute increases as temperature increases. If the boiling point of solvent is higher than that of solute, solubility of solute decreases as temperature increases.