What Happens To Heat During Phase Changes? How does melting and freezing occur and what is the role of heat during change of states?
On a hot summer day, we often use ice to cool a glass of water. When the ice melts, the water is cooled almost to the freezing temperature. If you set the glass where the sun shines on it, the water stays at about this same temperature until all the ice is gone. Radiant energy from the sun is being changed into heat in the glass of water. Yet the water does not get any warmer until all the ice is melted.
You have often used a thermometer to find the temperature of something. Thermometers show how hot or cold a material is. But they do not show how much heat was added to or removed from the material to make it reach this temperature. In the metric system, the amount of heat is measured in a unit called the calorie. A calorie is the amount of heat needed to raise the temperature of 1 gram of water 1° Centigrade. In the English system, the amount of heat is measured in the British Thermal Unit, abbreviated B.T.U. This is the amount of heat needed to raise the temperature of 1 pound of water 1° F.
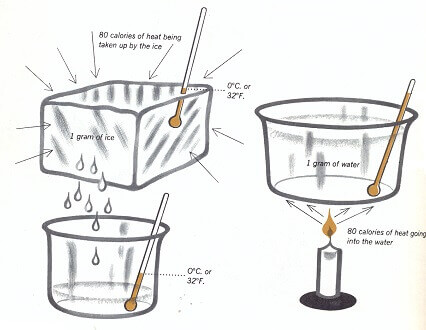
We are likely to think that heating always raises the temperature of things. But heating can also cause a change of state without raising the temperature of a material. When ice melts, heat is absorbed. This heat makes the molecules move about more freely so that they form a liquid. To change 1 gram of ice to water takes about 80 calories of heat. Instead of getting warmer, the wata stays at the same temperature as the ice until al of the ice is melted. If 80 calories of heat are then added to 1 gram of the water, it will be heated to 80° C., or 176° F. At this temperature, water is hot enough to burn your hand.
When any solid melts, heat is absorbed. This heat is taken from the materials around the melting solid. However, the amount of heat is not the same for all solids. For example, only about 35 calories are needed to melt 1 gram of paraffin. Ice uses more heat in melting than does almost any other solid. While each gram of ice is melting, it absorbs about 80 calories. So melting ice removes a large amount of heat from whatever is near it. When ice is put into water, the heat that melts the ice comes from the water. As this heat is given out, the water is cooled.

When heat is removed, water gets steadily colder until it reaches the freezing temperature. Then the water stays at this temperature until all of it is frozen.Though heat is still being removed, the water gets no colder. The same amount of heat needed to melt ice must be removed from water to freeze it. Then the molecules are held tightly together so that they form a solid. Each gram of water at the freezing temperature must give out about 80 calories before it can freeze. So freezing water gives out a large amount of heat to whatever is near it. While the last of this heat is coming out, the water stays at the freezing temperature but does not change to a solid. After all of the water is frozen, its temperature begins to fall again.