What does transmutation of elements mean? What is the history of transmutation of elements? Information about transmutation of elements.
Transmutation Of Elements conversion of any given chemical elements into other chemical elements. Transmutation of elements may appear to be something of a contradiction in terms, since an element is traditionally defined as a form of matter that cannot be changed into, or produced from, simpler forms of matter. It was the main purpose of medieval alchemists, who vainly attempted to convert base metals into gold. Their fruitless efforts depended on the concept of ancient Greek philosophers such as Empedocles, Aristotle, and others that all matter was composed of various proportions of the four “elements”: fire, water, earth, and air. With the development of modern chemistry in the 19th century, transmutation of the elements was deemed impossible. It later became a reality through the discovery of the phenomenon of radioactivity and subsequent insight into the structure of the atom and its nucleus.
The nature of a chemical element is determined by the electric charge of the nucleus of its atoms. Therefore, transmutation of an element involves a change of this charge. Because of the nal ture and magnitude of the forces that hold the atomic nucleus together and the shielding of the electrons surrounding the nucleus, the energy changes involved in ordinary chemical reactions do not affect the nuclear charge.
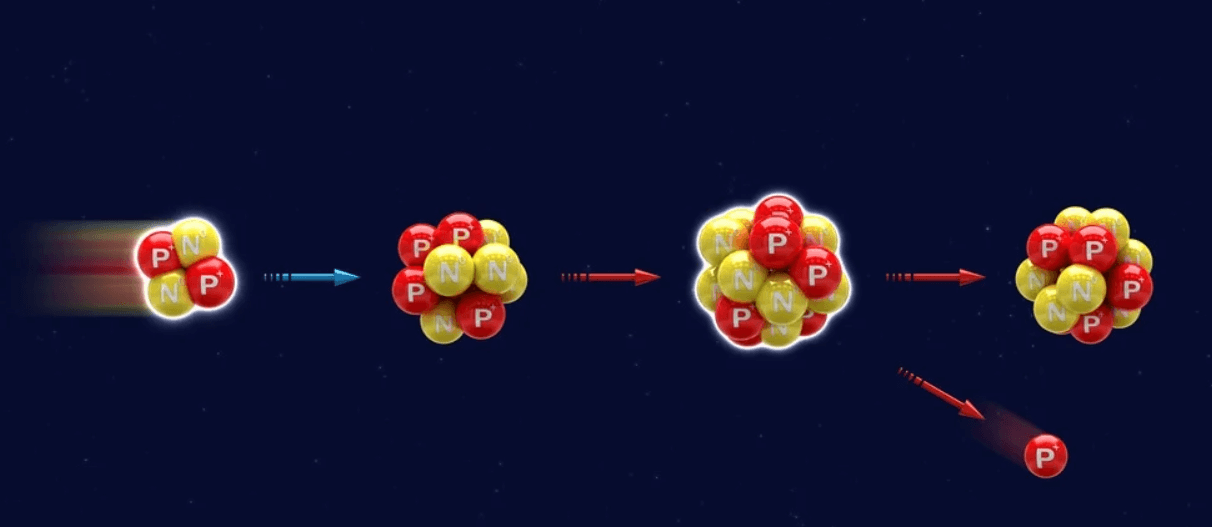
The first observed example of transmutation was caused by radioactivity, that is, the spontaneous ejection of charged particles from the nuclei of certain unstable atoms.
Artificially induced transmutation processes can be initiated by the entrance of ions, neutrons, or electromagnetic radiation into the nuclei. Because of the high electrostatic repulsive forces prevailing in the immediate neighborhood of the atomic nuclei, only ions possessing high energy can enter the nuclei. The uncharged neutrons require no excess energy to penetrate the nuclei and therefore can change only the mass but not the charge of the nucleus. Neutrons cause transmutation by knocking out a charged particle from the nucleus or by rendering the nucleus radioactive. Transmutations occurring under the influence of high energy electromagnetic radiation are referred to as phototransmutation or photodisintegration.

Ernest Rutherford (Source : wikipedia.org)
The first instance of artificial transmutation of a nonradioactive element was discovered by Ernest Rutherford in 1919 in the conversion of nitrogen to oxygen and hydrogen under the impact of high velocity helium nuclei ( a particles ) emitted by radioactive elements.
Entirely artificial transmutation was accomplished in 1932 by John D. Cockroft and Ernest T. S. Walton, by bombarding lithium and other nuclei with electrically accelerated protons, and by Norman Feather in England and by William D. Harkins and coworkers in the United States, by exposing nitrogen, oxygen, and other elements to fast neutrons, thereby electing a particles from the nuclei. Further progress in transmutation was a consequence of the discovery of artificial radioactivity induced by a particles (Irène and Frédéric Joliot-Curie, 1934) and by capture of slow neutrons (Enrico Fermi and coworkers, 1934).
The fission of uranium or thorium nuclei when subjected to the action of neutrons is a high energy transmutation process. In this process, the nucleus splits into two nuclei of approximately equal size, and a number of high energy neutrons are emitted. Fission was discovered by Otto Hahn and Fritz Strassmann in 1938 and correctly interpreted by Lise Meitner and Otto R. Frisch in 1939. Nuclear fission is the basis of the utilization of nuclear energy in the so-called atomic bomb and in nuclear power plants.
The advent of elaborate ion and electron accelerators ( cyclotrons, betatrons, synchrotrons ) and the availability of powerful neutron sources in nuclear reactors made the transmutation of virtually every element possible and led to the development of nuclear chemistry.
Transmutation or nuclear reactions are represented by formulas in which the superscript denotes the nuclear charge and the subscript indicates the atomic weight:
The products of transmutation reactions include radioactive isotopes of almost all elements, as well as certain elements themselves. Among the latter are the elements (numbered 43, 61, 85, and 87 ) which are missing from the periodic system and the so-called transuranium elements numbered 93 to 103, of which plutonium (number 94) has attained particular importance in connection with nuclear weapons.
mavi