Delve into the world of hydrocarbons as we explore their 10 fundamental characteristics, from their composition and energy content to their various forms and environmental impact. Gain insights into these essential compounds that play a vital role in numerous industries and our daily lives.
The hydrocarbons are a set of organic compounds. Its molecules are composed of carbon and hydrogen atoms, organized in different structures depending on the type of hydrocarbon. For the most part, hydrocarbons come from oil. This is because oil is the result of the decomposition of organic matter and therefore offers a large amount and concentration of carbon and hydrogen.
Petroleum derivatives, that is, hydrocarbons, are involved in many industries, from aeronautics to the toy industry. Almost all the fuels used in transportation are derived from hydrocarbons, which are used to create polluting waste (carbon dioxide). Therefore, it is currently trying to replace them with other types of fuels and energy sources. Hydrocarbons are a non-renewable resource, since they can not be manufactured by humans.

Characteristics Of Hydrocarbons
1. Organic molecular structure
All substances whose molecules contain carbon atoms forming a bond with other carbon molecules (carbon-carbon bond) and with hydrogen molecules (carbon-hydrogen bond) are considered organic.
Although each hydrocarbon has a distinctive molecule, they all share in their molecular structure a chain of carbon atoms each of which may be one in addition to one or more hydrogen atoms.
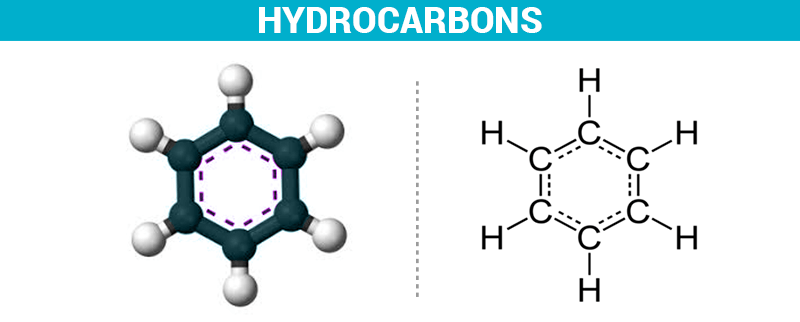
2. Aromatic hydrocarbons
The aromatic hydrocarbons have cyclic molecules, that is to say that the carbon atoms form a circle. There are double bonds between the carbon atoms. One of the aromatic hydrocarbons is benzene, as well as all its derivatives.
3. Aliphatic hydrocarbons
They are those hydrocarbons that do not form rings with double bonds, that is, they are not aromatic. The chains of the aliphatics can be opened or closed.
Open chain aliphatic hydrocarbons:
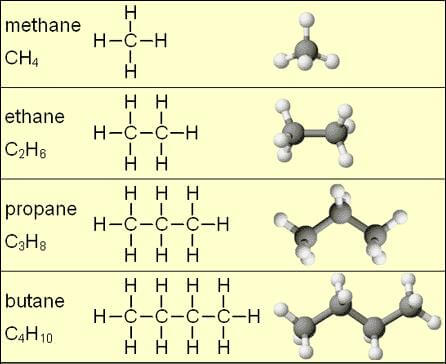
- Alkanes: The bonds between all the carbon atoms are simple. The molecular formula is CnH2n + 2 (n is the number of carbons).
- Alkenes: One of the bonds between the carbon atoms is double. The molecular formula is CnH2n.
- Alkynes: One of the bonds between the carbon atoms is triple. The molecular formula is CnH2n-2.
- Closed chain aliphatic hydrocarbons: They are called cyclic and the chains of the carbon atoms form rings, as in the case of aromatics. However, aliphatics do not have double bonds. They are called cycloalkanes.
4. Saturation
It is said that a substance is saturated when in its molecule all the carbon atoms are joined to other atoms by simple bonds. It is said that the molecule is saturated because the simple bonds can not be broken and therefore no more hydrogen atoms can be added.
The saturated hydrocarbons are the alkanes and the cycloalkanes. The unsaturated hydrocarbons are the aromatics, the alkenes and the alkynes.
5. Physical properties
- Boiling point: increases as the size of the alkane increases (the amount of carbon atoms). This is because the intermolecular forces are greater when the molecule is larger. For example, the boiling point of butane is -0.5 while the boiling point of nonane is 150.8.
- Density: also increases when the molecule is larger.
- Solubility: they are insoluble in water. This is because they are polar substances, that is to say that the electrical charges of each molecule are separated.
6. Chemical properties
- Fuels: all hydrocarbons can reach complete oxidation. They begin to oxidize in the presence of oxygen or a source of heat. One of the substances resulting from combustion is carbon dioxide. That is why hydrocarbons are polluting substances when used as fuel.
- Pyrolysis: when exposed to alkanes at a heat of 800 degrees can decompose forming alkenes and free hydrogen.
- Halogenation: under the presence of light with ultraviolet rays, the alkanes react together with the halogens, producing derivatives of the allogens.
7. Applications
The hydrocarbons are mainly used as fuel for transport and industry, but also in electric generators. In addition, they are the raw material for lubricants and greases for vehicles, as well as asphalts.
The hydrocarbons are processed to manufacture all kinds of plastics, acrylics, nylon, gloves, paints, synthetic fibers, containers, adhesives, insecticides, detergents, refrigerants and fertilizers.
8. Degradation
Hydrocarbons are contaminants not only from the waste produced when they are burned, but also when the oil spills on the land or in the water. Despite being an organic substance, hydrocarbons are not usually biodegradable.
However, research has been developed trying to solve this problem. For this, a combination of bacteria that degrade the hydrocarbon molecules in chain is used: that is, a bacterium manages to break the molecule to make it “edible” for another bacterium.
9. Nomenclature
To name hydrocarbons, a series of prefixes (at the beginning of the name) and suffixes (at the end of the name) are used to indicate the number of bonds and atoms.
Examples of prefixes according to the number of carbon atoms: Met, Et, Prop, But, Pent, Hex, Hept, Oct, Non.
10. Examples of Hydrocarbons
- Methane: CH4 (alkane)
- Ethane: CH3 – CH3 (alkane)
- Propane: CH3 – CH2 – CH3 (alkane)
- Butane: CH3 – CH2 – CH2 – CH3 (alkane)
- Butino: CH ≡ C – CH2CH3 (alkyne)
- Heptino: CH3 – CH2 – C ≡ C – CH2 – CH3 (alkyne)